Therapeutic Drug Monitoring
Anti-Cetuximab (Erbitux®)ADA Quantitative ELISA Kit
- SKU:
- HUMB00035
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Quantitative
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Cetuximab (Erbitux®)
- Research Area:
- Anti-Cancer
Description
Anti-Cetuximab (Erbitux®) ADA Quantitative ELISA Kit
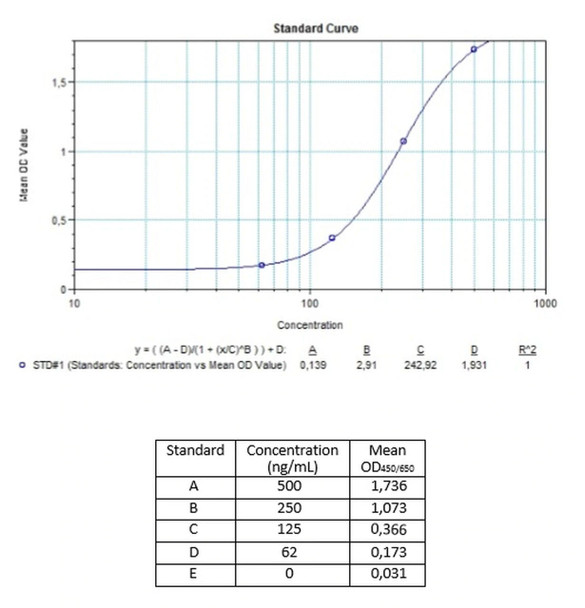
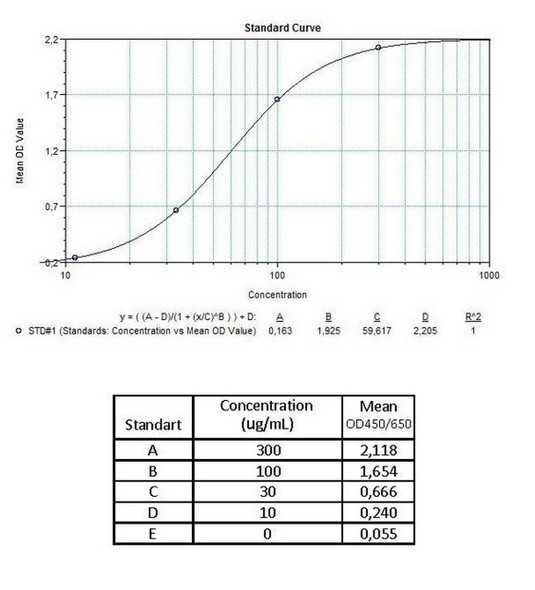
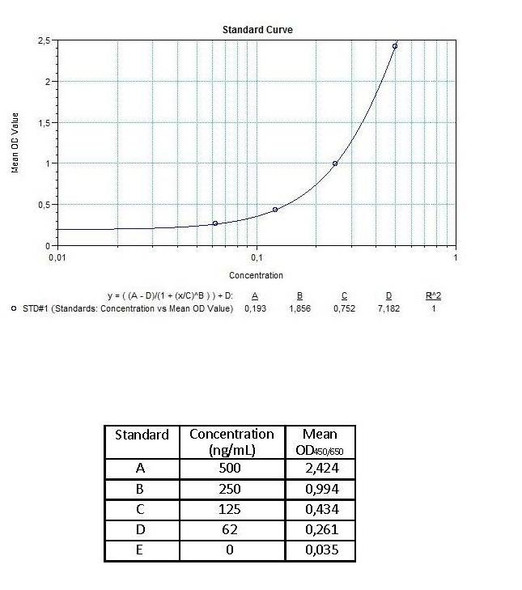
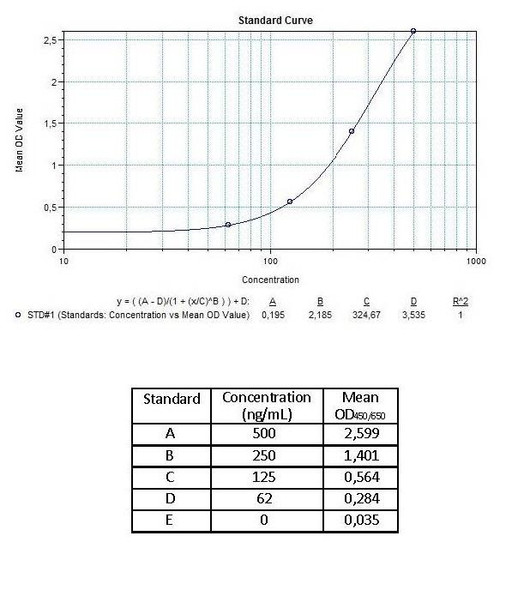
Enzyme-linked immunosorbent assay for the quantitative determination of antibodies to cetuximab (Erbitux®) in serum and plasma. Cetuximab (Erbitux®) was associated to the development of anti-cetuximab antibodies, with some reported to be neutralizing patients. The Assay Genie Anti-Cetuximab (Erbitux®) ADA Quantitative ELISA Kit can be efficiently used for monitoring Cetuximab anti-drug antibodies in biological samples and is for research use only.
Anti-Cetuximab (Erbitux®) ADA Quantitative ELISA Kit test principle
The Assay Genie Antibody to cetuximab (Erbitux®) ELISA is a sandwich assay for the determination of antibodies against cetuximab in serum and plasma samples. During the first incubation period, antibodies to cetuximab (ATC) in patient serum/ plasma samples are captured by the drug cetuximab (Erbitux®) coated on the wall of the microtiter wells. After washing away the unbound components from samples, a peroxidase-labelled specific conjugate is added to each well and then incubated. After a second washing step, the bound enzymatic activity is detected by addition of tetramethylbenzidine (TMB) chromogen-substrate. Finally, the reaction is terminated with an acidic stop solution. The intensity of the reaction colour is directly proportional to the concentration of ATC in sample.
Anti-Cetuximab (Erbitux®) ADA Quantitative ELISA Kit product information
| Information | Description |
| Application | Free drug |
| Required Volume (μl) | 10 |
| Total Time (min) | 140 |
| Sample Type | Serum, Plasma |
| Number of Assays | 96 |
| Detection Limit (ng/mL) | 30 (ng/mL) |
| Spike Recovery (%) | 85-115% |
| Shelf Life (year) | 1 |
| Alternative Names | Anti-Epidermal Growth Factor Receptor (EGFR) mAb Erbitux |
Anti-Cetuximab (Erbitux®) ADA Quantitative ELISA - Key Information
Cetuximab (Erbitux®) mode of action
Cetuximab (IMC-C225, Erbitux) is a human/murine chimeric monoclonal antibody of the immunoglobulin G1 (IgG1) and FDA-approved epidermal growth factor receptor (EGFR) inhibitor. It is a 152 kDa protein composed of four polypeptide chain. There are 32 cysteine residues forming accordingly 16 potential disulfide bonds.
Cetuximab (ICM-225, Erbitux™) works by binding to the extracellular domain III of EGFR. This binding partially blocks the EGFR ligand-binding domain and sterically hinders the correct extended conformation of the dimerization arm on domain II. Cetuximab therefore prevents both ligand binding and the proper exposure of the EGFR dimerization domain, which hinders dimerization with other HER family members.
Cetuximab (Erbitux®) uses
Preclinical studies have shown that cetuximab enhances the anti-tumour effects of chemotherapy (e.g. that of irinotecan in colorectal cancer) as well as radiotherapy (e.g. in squamous cell carcinoma of the head and neck) by inhibiting cell proliferation, angiogenesis and metastasis and by promoting apoptosis is used for the treatment of metastatic colorectal cancer, metastatic non-small cell lung cancer and head and neck cancer.
Cetuximab blocks growth factor-induced activation of the downstream mitogen-activated protein kinase, inhibiting cell proliferation. Cetuximab also increases the receptor internalization which is another mechanism to silence the receptor. Cetuximab arrests cell cycle at G1 gap phase by upregulating antiproliferative p27 kip1 , which functions via complex formation with Cdk2, and downregulating proliferating cell nuclear antigen (PCNA).
Cetuximabdecreases angiogenic factors, inhibits tumor-cell invasion and metastasis via downregulation of matrix metalloproteinases (MMPs) and VEGF, and promotes apoptosis by upregulating apoptotic protein, Bax, with the help of other chemotherapeutic agents. Cetuximab has been widely shown to display synergistic effect with other agents and/or radiotherapy. Binding of antigen-binding fragment (Fab) of Cetuximab, which displays higher affinity comparing to ligands of EGFR, takes place via domain III of extracellular EGFR, preventing the receptor from conformational change to be dimerized and blocking EGFR signalling through inhibition of EGF and TGF-alpha-stimulated phosphorylation of the receptor.
Cetuximab (Erbitux®) pharmacokinetics and pharmacodynamics
Pharmacokinetics and Pharmacodynamics In a study conducted by Fracasso et al., patients with colorectal, breast, and head and neck carcinomas were administered with one of different dosages of cetuximab (50, 100, 250, 400 and 500 mg/m2 ). For each concentration, cetuximab serum concentration was showed to reach maximum at 3h and decrease slowly. Serum concentration decreased to baseline at 96 h and 168 h for dosages 50 and 100 mg/m2 , respectively. Mean 4 maximum observed concentrations (Cmax) increased in a dose dependent manner (from 22.8 ug/ml to 245.6 ug/ml). It was indistinguishable for 400 mg/m2 (Cmax=228.9 ug/ml) and 500 mg/m2 (Cmax=245.6 ug/ml).
The mean total body clearance based on body surface area for cetuximab was similar following doses of >100 mg/m2 (range, 34.4-19.3 L/h/m2 ) but greater in the 50 mg/m2 dose group (65.9 L/h/m2 ). Biopsy results showed that maximal cytoplasmic EGFR downregulation after treatment was seen in 8 h with 400 mg/m2 dosage. After 250 mg/m2 weekly cetuximab administration, the average trough level of patients with both partial responses (PRs) and stable disease (SD) was 60,742 ng/ml (~400 nmol/l) compared with those patients with progressive disease (PD; 33,208 ng/ml).
In another study, cetuximab was infused as loading dose of 400 mg/m2 followed by weekly infusions of 250 mg/m2 in colorectal cancer patients. Median residual concentrations were 41 and 54 mg/L on days 14 and 28, respectively. It was determined that initial serum albumin concentration was significantly related to first-order elimination clearance of cetuximab. Central volume of distribution was 2.96 L (4%), peripheral volume of distribution was 4.65 L (6%), elimination clearance was 0.479 L/d (4%) and distribution clearance was 0.836 L/d (8%).
Cetuximab (Erbitux®) and Epidermal Growth Factor Receptor
Epidermal growth factor receptor (EGFR) Epidermal growth factor receptor (EGFR; HER1; ErbB1) is a transmembrane tyrosine kinase encoded by c-erb-B proto-oncogene, expressed in normal and malignant cells and stimulated by epidermal growth factor (EGF) or transforming growth factor-alpha (TGF-alpha) binding extracellular domain of the receptor, leading receptor to dimerize and activating intracellular kinase domain on each receptor, bringing about phosphorylation of tyrosine residues on each member of the receptor pair. Next, signalling complexes form in cytoplasm to activate gene transcription responding for such as cell proliferation. Termination of signalling occurs through internalization of receptor-ligand complex. Activation of EGFR results in perturbation of mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase, and AKT pathways triggering tumorigenic processes, such as increased proliferation, angiogenesis and metastasis, and prevents apoptosis.
Breast, lung, colon, prostate, kidney, bladder, head and neck, and ovary cancers have been associated to EGFR overexpression which causes early disease progression, poor survival, and resistance to chemotherapy in many epithelial malignancies. Epidermal growth factor receptor/human epidermal growth factor receptor 1 (EGFR/HER1) and its ligand, transforming growth factor alpha (TGF-alpha) were showed to involve in hepatocarcinogenesis. EGFR is overexpressed in hepatocellular carcinoma (HCC). To overcome the uncontrollable effect of EGFR triggering cancer development, monoclonal antibodies, such as Cetuximab, have been shown to be used as blockers in vitro and in vivo.
Cetuximab (Erbitux®) immunogenicity
As with any biologic therapeutic, immunogenicity, in the form of anti-cetuximab antibodies can occur. The demonstration of anti-cetuximab antibodies during treatment with cetuximab (Erbitux®) is a major concern. The Assay Genie Anti-Cetuximab (Erbitux®) ADA Quantitative ELISA Kit can be efficiently used for monitoring Cetuximab anti-drug antibodies in biolgical samples and is for research use only.
Anti-Cetuximab (Erbitux®) ADA Quantitative ELISA Kit Contents
| Size | Kit Contents |
| 1x12x8 | Microtiter Plate Break apart strips. Microtiter plate with 12 rows each of 8 wells coated with reactant. |
| 7 x 1 mL | ATC Standards A-E, High Level Control, Low Level Control |
| 1 x 50 mL | Assay Buffer |
| 1 x 12 mL | Semi-Log Graph Paper |
| 1 x 12 mL | Peroxidase Conjugate |
| 1 x 12 mL | TMB Substrate Solution |
| 1 x 12 mL | TMB Stop Solution |
| 1 x 50 mL | Wash Buffer concentrate (20x) |
| 2 x 1 | Adhesive Foil |
Anti-Cetuximab (Erbitux®) ADA Quantitative ELISA Protocol
| Steps | Protocol |
| 1 | QUANTITATIVE ELISA TEST FORMAT Wells: |
| 2 | Cover the plate with adhesive film. Briefly mix contents by gently shaking the plate. Incubate 60 min at room temperature (18-25°C). |
| 3 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300µL of diluted. Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
| 4 | Pipette 100 µL of ready-to use Peroxidase Conjugate into each well. |
| 5 | Cover the plate with adhesive foil. Incubate 60 min at room temperature (18- 25°C). |
| 6 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300 µL of diluted Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
| 7 | Pipette 100 µL of TMB Substrate Solution into each well. |
| 8 | Incubate 20 min (without adhesive foil) at room temperature (18-25°C) in the dark |
| 9 | Stop the substrate reaction by adding 100 µL of Stop Solution into each well. Briefly mix contents by gently shaking the plate. Colour changes from blue to yellow. |
| 10 | Measure optical density with a photometer at 450/650 nm within 30 min after pipetting of the Stop Solution. |
Trademarks
Erbitux® CETUXIMAB is a trademark of ImClone LLC